SteamLife® Joint Renewal Protocol
A Transformative Approach to Treat Joint Pain and Osteoarthritis
Learn how umbilical cord stem cell and exosome therapies are redefining the possibilities for chronic pain treatment.
Addressing Joint Pain with Regenerative Medicine
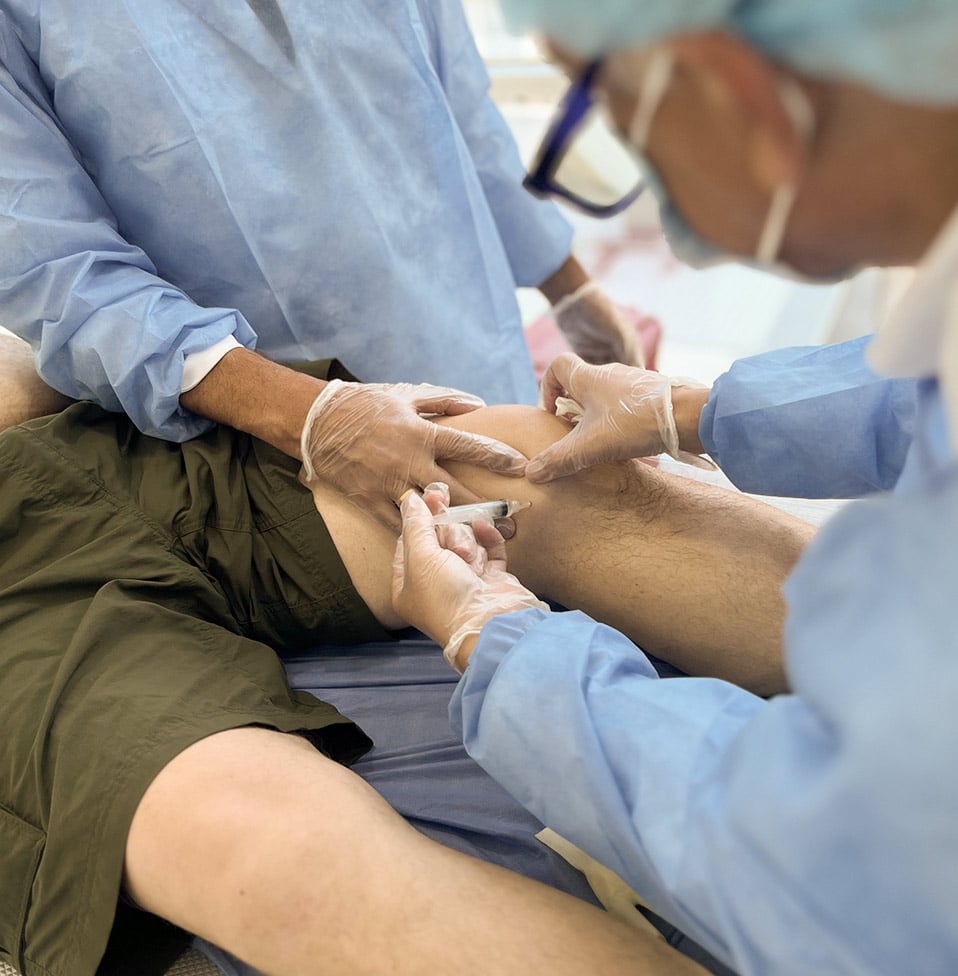
For individuals experiencing chronic joint pain and reduced mobility due to osteoarthritis or other degenerative conditions, alternative treatment options are now available. Umbilical Cord Mesenchymal Stem Cell (U-MSC) and Exosome therapies represent a new approach to managing joint pain, offering innovative and minimally invasive treatment solutions.
These therapies harness the body’s natural regenerative abilities to promote tissue repair and regeneration within affected joints. By targeting damaged areas, they help reduce inflammation and support the restoration of joint structure and function, paving the way for improved mobility and quality of life.
Targeted Regeneration
U-MSCs support the repair and regeneration of joint cartilage and tissue, addressing the underlying damage from osteoarthritis.

Natural Anti-Inflammatory
Exosomes from U-MSCs deliver anti-inflammatory signals that help reduce swelling and relieve joint pain.
Enhanced Mobility
By improving joint function and cushioning, U-MSC therapies can lead to better mobility and quality of life with minimal downtime.
Advancing the Management of Joint Pain
StemLife® Joint Renewal Protocol leverages the combined use of U-MSCs and derived exosomes to treat joint disorders. This integrated approach specifically targets inflammation and tissue degeneration, promoting joint regeneration.
U-MSC
At the forefront of regenerative medicine, U-MSC offer a powerful, non-invasive solution for chronic joint pain. Their ability to rejuvenate and repair damaged joint tissues is transforming how we treat conditions like osteoarthritis, providing a pathway to improved mobility and a better quality of life.
U-MSC Derived Exosomes
Exosomes, tiny vesicles released by U-MSCs, play a crucial role in cell communication and tissue healing. These exosomes carry signals that help reduce inflammation and promote tissue repair, making them essential in restoring joint function.
The synergy between U-MSC and exosome treatments offers a new, scientifically-backed approach to managing joint pain. These therapies harness the body’s natural healing processes, providing long-lasting relief without invasive procedures.
Anti-inflammatory Effects
Chronic joint pain often arises from persistent inflammation. Components of regenerative medicine, like growth factors and cytokines, can modulate inflammatory responses, reducing pain and swelling in the affected areas.
Regenerative Capacity
U-MSC possess the capacity to differentiate into multiple cell types, encompassing both bone and cartilage. Upon administration into a damaged joint, these cells have the potential to facilitate tissue repair, contributing to the regeneration of the joint.
Tissue Engineering
Another facet of regenerative medicine is tissue engineering, creating functional tissue constructs that can replace or improve damaged or degenerated tissues.
Synovial fluid Improvement
Synovial fluid is the lubricating fluid in our joints. U-MSC therapy might help in enhancing the characteristics of this fluid, thus providing better joint mobility and less pain.
Comprehensive Joint Healing: Targeting Inflammation, and Mobility
Evidence-based Care: Our treatments are rooted in scientific research, aimed at offering relief through the body’s natural regenerative mechanisms.
Personalized Treatment: Every therapy plan is tailored to your specific needs, targeting inflammation and joint degeneration with precision.
Long-term Wellness: Beyond temporary relief, our goal is to support sustainable joint health and recovery.
Wang, Hao-Nan; Rong, Xiao; Yang, Lu-Ming; Hua, Wei-Zhong; Nicorresponding, Guo-Xin
Advances in Stem Cell Therapies for Rotator Cuff Injuries Journal Article
In: 2022.
@article{nokey,
title = {Advances in Stem Cell Therapies for Rotator Cuff Injuries},
author = {Hao-Nan Wang and Xiao Rong and Lu-Ming Yang and Wei-Zhong Hua and Guo-Xin Nicorresponding},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9174670/},
year = {2022},
date = {2022-05-25},
abstract = {Rotator cuff injury is a common upper extremity musculoskeletal disease that may lead to persistent pain and functional impairment. Despite the clinical outcomes of the surgical procedures being satisfactory, the repair of the rotator cuff remains problematic, such as through failure of healing, adhesion formation, and fatty infiltration. Stem cells have high proliferation, strong paracrine action, and multiple differentiation potential, which promote tendon remodeling and fibrocartilage formation and increase biomechanical strength. Additionally, stem cell-derived extracellular vesicles (EVs) can increase collagen synthesis and inhibit inflammation and adhesion formation by carrying regulatory proteins and microRNAs. Therefore, stem cell-based therapy is a promising therapeutic strategy that has great potential for rotator cuff healing. In this review, we summarize the advances of stem cells and stem cell-derived EVs in rotator cuff repair and highlight the underlying mechanism of stem cells and stem cell-derived EVs and biomaterial delivery systems. Future studies need to explore stem cell therapy in combination with cellular factors, gene therapy, and novel biomaterial delivery systems.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ding, Guocheng; Du, Jianing; Hu, Xiaoqing; Yingfang,
Mesenchymal Stem Cells From Different Sources in Meniscus Repair and Regeneration Journal Article
In: 2022.
@article{nokey,
title = {Mesenchymal Stem Cells From Different Sources in Meniscus Repair and Regeneration},
author = {Guocheng Ding and Jianing Du and Xiaoqing Hu and Yingfang},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9091333/},
year = {2022},
date = {2022-04-27},
urldate = {2022-04-27},
abstract = {Meniscus damage is a common trauma that often arises from sports injuries or menisci tissue degeneration. Current treatment methods focus on the repair, replacement, and regeneration of the meniscus to restore its original function. The advance of tissue engineering provides a novel approach to restore the unique structure of the meniscus. Recently, mesenchymal stem cells found in tissues including bone marrow, peripheral blood, fat, and articular cavity synovium have shown specific advantages in meniscus repair. Although various studies explore the use of stem cells in repairing meniscal injuries from different sources and demonstrate their potential for chondrogenic differentiation, their meniscal cartilage-forming properties are yet to be systematically compared. Therefore, this review aims to summarize and compare different sources of mesenchymal stem cells for meniscal repair and regeneration.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Stewart, Claire E.
Stem cells and regenerative medicine in sport science Journal Article
In: 2021.
@article{nokey,
title = {Stem cells and regenerative medicine in sport science},
author = {Claire E. Stewart},
year = {2021},
date = {2021-08-27},
abstract = {The estimated cost of acute injuries in college-level sports in the USA is ∼1.5 billion dollars annually, without considering the cost of follow-up rehabilitation. In addition to this huge financial burden, sports injuries may be career-ending for some athletes without appropriate diagnosis and relevant interventions. With a growing number of females participating in contact-based and pivoting sports, middle-aged individuals returning to sports,s and natural injuries of aging all increasing, such costs and negative implications for quality of life will expand. For those injuries that cannot be predicted and prevented, there is a real need to optimize repair, recovery, and function post-injury in the sporting and clinical worlds. The 21st century has seen rapid growth in regenerative medicine for sporting injuries to progress recovery and facilitate return to sport. Such interventions harness knowledge relating to stem cells as a potential for injury repair. While the field is rapidly growing, consideration beyond the stem cells to the factors they secrete should be considered in developing practical, affordable treatments.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xu, Yue; Zhang, Wan-Xia; Wang, Li-Na; Ming, Yue-Qing; Li, Yu-Lin; Ni, Guo-Xin
Stem cell therapies in tendon-bone healing Journal Article
In: 2021.
@article{nokey,
title = {Stem cell therapies in tendon-bone healing},
author = {Yue Xu and Wan-Xia Zhang and Li-Na Wang and Yue-Qing Ming and Yu-Lin Li and Guo-Xin Ni},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8316867/},
year = {2021},
date = {2021-07-26},
abstract = {Tendon-bone insertion injuries such as rotator cuff and anterior cruciate ligament injuries are currently highly common and severe. The key method of treating this kind of injury is the reconstruction operation. The success of this reconstructive process depends on the ability of the graft to incorporate into the bone. Recently, there has been substantial discussion about how to enhance the integration of tendon and bone through biological methods. Stem cells like bone marrow mesenchymal stem cells (MSCs), tendon stem/progenitor cells, synovium-derived MSCs, adipose-derived stem cells, or periosteum-derived periosteal stem cells can self-regenerate and potentially differentiate into different cell types, which have been widely used in tissue repair and regeneration. Thus, we concentrate in this review on the current circumstances of tendon-bone healing using stem cell therapy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Khan, Shujhat; Mafi, Pouya; Mafi, Reza; Khan, Wasim
A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion Journal Article
In: 2018.
@article{nokey,
title = {A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion},
author = {Shujhat Khan and Pouya Mafi and Reza Mafi and Wasim Khan},
url = {https://doi.org/10.2174/1574888x11666170907120030},
doi = {https://doi.org/10.2174/1574888x11666170907120030},
year = {2018},
date = {2018-05-01},
urldate = {2018-05-01},
abstract = {Spinal surgery presents a challenge for both neurosurgery and orthopaedic surgery. Due to the heterogeneous differentiation potential of mesenchymal stem cells, there is much interest in the treatment of spine surgery. Animal and human trials focussing on the efficacy of mesenchymal stem cells in spinal cord injury, spine fusion and disc degeneration were included in this systematic review. Published articles up to January 2016 from MEDLINE, PubMed and Ovid were used by searching for specific terms. Of the 2595 articles found, 53 met the selection criteria and were included for analysis (16 on spinal cord injury, 28 on intervertebral disc repair and 9 on spinal fusion). Numerous studies reported better results when the mesenchymal stem cells were used in co-culture with other cells or used in scaffolds. Mesenchymal stem cells were also found to have an immune-modulatory role, which can improve surgical outcome. This systematic review suggests that mesenchymal stem cells can be used safely and effectively for these spinal surgery treatments. Whilst, in certain studies, mesenchymal stem cells did not necessarily show improved results from existing treatments, they provide an alternative option. This can reduce morbidity that arises from current surgical treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ajibadea, David A.; Vance, Danica D.; Harea, Joshua M.; Lesniak, Lee D. Kaplanand Bryson P.
Emerging Applications of Stem Cell and Regenerative Medicine to Sports Injuries Journal Article
In: 2014.
@article{nokey,
title = {Emerging Applications of Stem Cell and Regenerative Medicine to Sports Injuries},
author = {David A. Ajibadea and Danica D. Vance and Joshua M. Harea and Lee D. Kaplanand Bryson P. Lesniak},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4555618/},
year = {2014},
date = {2014-02-02},
abstract = {Cell-based therapies and regenerative medicine offer safe and potentially efficacious treatment for sports-related musculoskeletal injuries. Basic science and preclinical studies that support the possibility of enhanced recovery from sports injuries using cell-based therapies are accumulating; however, more clinical evidence is necessary to define the indications and parameters for their use. Accordingly, exposing patients to cell-based therapies could confer an unacceptable risk profile with minimal or no benefit. Continued clinical testing with animal models and clinical trials is necessary to determine the relative risks and benefits as well as the indications and methodology of treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ennis, William J.; Sui, Audrey; Bartholomew, Amelia
Stem Cells and Healing: Impact on Inflammation Journal Article
In: 2013.
@article{nokey,
title = {Stem Cells and Healing: Impact on Inflammation},
author = {William J. Ennis and Audrey Sui and Amelia Bartholomew},
url = {https://doi.org/10.1016/j.fsc.2018.06.004},
doi = {https://doi.org/10.1016/j.fsc.2018.06.004},
year = {2013},
date = {2013-09-02},
urldate = {2013-09-02},
abstract = {Multipotent stem cells have paved the way for new applications and deeper understanding in regenerative medicine and the pathophysiology of aging. During skin aging, cumulative photodamage, exhaustion of endogenous stem cell populations, mechanical stress, and increased fibrosis lead to skin with decreased epidermal thickness and compromised dermal integrity. Specific stem cells in the hair follicle create new keratinocytes after activation by defensin peptides, released by neutrophils during wounding. Studies pertaining to defensin peptides' efficacy on skin aging have been published, highlighting their potential as a new therapy for skin rejuvenation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Wang, Xiuli Cong Lihua Wang Liming
Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy Journal Article
In: 2013.
@article{nokey,
title = {Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy},
author = {Xiuli Cong Lihua Wang Liming Wang},
url = {https://doi.org/10.1089/scd.2013.0023},
year = {2013},
date = {2013-08-13},
urldate = {2013-08-13},
abstract = {This study was designed to assess the safety and efficacy of human umbilical cord mesenchymal stem cells (UC-MSCs) in the treatment of rheumatoid arthritis (RA). In this ongoing cohort, 172 patients with active RA who had inadequate responses to traditional medication were enrolled. Patients were divided into two groups for different treatment: disease-modifying anti-rheumatic drugs (DMARDs) plus medium without UC-MSCs, or DMARDs plus UC-MSCs group (4×107 cells per time) via intravenous injection. Adverse events and the clinical information were recorded. Tests for serological markers to assess safety and disease activity were conducted. Serum levels of inflammatory chemokines/cytokines were measured, and lymphocyte subsets in peripheral blood were analyzed. No serious adverse effects were observed during or after infusion. The serum levels of tumor necrosis factor-alpha and interleukin-6 decreased after the first UC-MSCs treatment (P<0.05). The percentage of CD4+CD25+Foxp3+ regulatory T cells of peripheral blood was increased (P<0.05). The treatment induced a significant remission of disease according to the American College of Rheumatology improvement criteria, the 28-joint disease activity score, and the Health Assessment Questionnaire. The therapeutic effects maintained for 3–6 months without continuous administration, correlating with the increased percentage of regulatory T cells of peripheral blood. Repeated infusion after this period can enhance the therapeutic efficacy. In comparison, there were no such benefits observed in control group of DMARDS plus medium without UC-MSCs. Thus, our data indicate that treatment with DMARDs plus UC-MSCs may provide safe, significant, and persistent clinical benefits for patients with active RA.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Wang, Hao-Nan; Rong, Xiao; Yang, Lu-Ming; Hua, Wei-Zhong; Nicorresponding, Guo-Xin
Advances in Stem Cell Therapies for Rotator Cuff Injuries Journal Article
In: 2022.
@article{nokey,
title = {Advances in Stem Cell Therapies for Rotator Cuff Injuries},
author = {Hao-Nan Wang and Xiao Rong and Lu-Ming Yang and Wei-Zhong Hua and Guo-Xin Nicorresponding},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9174670/},
year = {2022},
date = {2022-05-25},
abstract = {Rotator cuff injury is a common upper extremity musculoskeletal disease that may lead to persistent pain and functional impairment. Despite the clinical outcomes of the surgical procedures being satisfactory, the repair of the rotator cuff remains problematic, such as through failure of healing, adhesion formation, and fatty infiltration. Stem cells have high proliferation, strong paracrine action, and multiple differentiation potential, which promote tendon remodeling and fibrocartilage formation and increase biomechanical strength. Additionally, stem cell-derived extracellular vesicles (EVs) can increase collagen synthesis and inhibit inflammation and adhesion formation by carrying regulatory proteins and microRNAs. Therefore, stem cell-based therapy is a promising therapeutic strategy that has great potential for rotator cuff healing. In this review, we summarize the advances of stem cells and stem cell-derived EVs in rotator cuff repair and highlight the underlying mechanism of stem cells and stem cell-derived EVs and biomaterial delivery systems. Future studies need to explore stem cell therapy in combination with cellular factors, gene therapy, and novel biomaterial delivery systems.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ding, Guocheng; Du, Jianing; Hu, Xiaoqing; Yingfang,
Mesenchymal Stem Cells From Different Sources in Meniscus Repair and Regeneration Journal Article
In: 2022.
@article{nokey,
title = {Mesenchymal Stem Cells From Different Sources in Meniscus Repair and Regeneration},
author = {Guocheng Ding and Jianing Du and Xiaoqing Hu and Yingfang},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9091333/},
year = {2022},
date = {2022-04-27},
urldate = {2022-04-27},
abstract = {Meniscus damage is a common trauma that often arises from sports injuries or menisci tissue degeneration. Current treatment methods focus on the repair, replacement, and regeneration of the meniscus to restore its original function. The advance of tissue engineering provides a novel approach to restore the unique structure of the meniscus. Recently, mesenchymal stem cells found in tissues including bone marrow, peripheral blood, fat, and articular cavity synovium have shown specific advantages in meniscus repair. Although various studies explore the use of stem cells in repairing meniscal injuries from different sources and demonstrate their potential for chondrogenic differentiation, their meniscal cartilage-forming properties are yet to be systematically compared. Therefore, this review aims to summarize and compare different sources of mesenchymal stem cells for meniscal repair and regeneration.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Stewart, Claire E.
Stem cells and regenerative medicine in sport science Journal Article
In: 2021.
@article{nokey,
title = {Stem cells and regenerative medicine in sport science},
author = {Claire E. Stewart},
year = {2021},
date = {2021-08-27},
abstract = {The estimated cost of acute injuries in college-level sports in the USA is ∼1.5 billion dollars annually, without considering the cost of follow-up rehabilitation. In addition to this huge financial burden, sports injuries may be career-ending for some athletes without appropriate diagnosis and relevant interventions. With a growing number of females participating in contact-based and pivoting sports, middle-aged individuals returning to sports,s and natural injuries of aging all increasing, such costs and negative implications for quality of life will expand. For those injuries that cannot be predicted and prevented, there is a real need to optimize repair, recovery, and function post-injury in the sporting and clinical worlds. The 21st century has seen rapid growth in regenerative medicine for sporting injuries to progress recovery and facilitate return to sport. Such interventions harness knowledge relating to stem cells as a potential for injury repair. While the field is rapidly growing, consideration beyond the stem cells to the factors they secrete should be considered in developing practical, affordable treatments.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Xu, Yue; Zhang, Wan-Xia; Wang, Li-Na; Ming, Yue-Qing; Li, Yu-Lin; Ni, Guo-Xin
Stem cell therapies in tendon-bone healing Journal Article
In: 2021.
@article{nokey,
title = {Stem cell therapies in tendon-bone healing},
author = {Yue Xu and Wan-Xia Zhang and Li-Na Wang and Yue-Qing Ming and Yu-Lin Li and Guo-Xin Ni},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8316867/},
year = {2021},
date = {2021-07-26},
abstract = {Tendon-bone insertion injuries such as rotator cuff and anterior cruciate ligament injuries are currently highly common and severe. The key method of treating this kind of injury is the reconstruction operation. The success of this reconstructive process depends on the ability of the graft to incorporate into the bone. Recently, there has been substantial discussion about how to enhance the integration of tendon and bone through biological methods. Stem cells like bone marrow mesenchymal stem cells (MSCs), tendon stem/progenitor cells, synovium-derived MSCs, adipose-derived stem cells, or periosteum-derived periosteal stem cells can self-regenerate and potentially differentiate into different cell types, which have been widely used in tissue repair and regeneration. Thus, we concentrate in this review on the current circumstances of tendon-bone healing using stem cell therapy.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Khan, Shujhat; Mafi, Pouya; Mafi, Reza; Khan, Wasim
A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion Journal Article
In: 2018.
@article{nokey,
title = {A Systematic Review of Mesenchymal Stem Cells in Spinal Cord Injury, Intervertebral Disc Repair and Spinal Fusion},
author = {Shujhat Khan and Pouya Mafi and Reza Mafi and Wasim Khan},
url = {https://doi.org/10.2174/1574888x11666170907120030},
doi = {https://doi.org/10.2174/1574888x11666170907120030},
year = {2018},
date = {2018-05-01},
urldate = {2018-05-01},
abstract = {Spinal surgery presents a challenge for both neurosurgery and orthopaedic surgery. Due to the heterogeneous differentiation potential of mesenchymal stem cells, there is much interest in the treatment of spine surgery. Animal and human trials focussing on the efficacy of mesenchymal stem cells in spinal cord injury, spine fusion and disc degeneration were included in this systematic review. Published articles up to January 2016 from MEDLINE, PubMed and Ovid were used by searching for specific terms. Of the 2595 articles found, 53 met the selection criteria and were included for analysis (16 on spinal cord injury, 28 on intervertebral disc repair and 9 on spinal fusion). Numerous studies reported better results when the mesenchymal stem cells were used in co-culture with other cells or used in scaffolds. Mesenchymal stem cells were also found to have an immune-modulatory role, which can improve surgical outcome. This systematic review suggests that mesenchymal stem cells can be used safely and effectively for these spinal surgery treatments. Whilst, in certain studies, mesenchymal stem cells did not necessarily show improved results from existing treatments, they provide an alternative option. This can reduce morbidity that arises from current surgical treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ajibadea, David A.; Vance, Danica D.; Harea, Joshua M.; Lesniak, Lee D. Kaplanand Bryson P.
Emerging Applications of Stem Cell and Regenerative Medicine to Sports Injuries Journal Article
In: 2014.
@article{nokey,
title = {Emerging Applications of Stem Cell and Regenerative Medicine to Sports Injuries},
author = {David A. Ajibadea and Danica D. Vance and Joshua M. Harea and Lee D. Kaplanand Bryson P. Lesniak},
url = {https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4555618/},
year = {2014},
date = {2014-02-02},
abstract = {Cell-based therapies and regenerative medicine offer safe and potentially efficacious treatment for sports-related musculoskeletal injuries. Basic science and preclinical studies that support the possibility of enhanced recovery from sports injuries using cell-based therapies are accumulating; however, more clinical evidence is necessary to define the indications and parameters for their use. Accordingly, exposing patients to cell-based therapies could confer an unacceptable risk profile with minimal or no benefit. Continued clinical testing with animal models and clinical trials is necessary to determine the relative risks and benefits as well as the indications and methodology of treatment.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Ennis, William J.; Sui, Audrey; Bartholomew, Amelia
Stem Cells and Healing: Impact on Inflammation Journal Article
In: 2013.
@article{nokey,
title = {Stem Cells and Healing: Impact on Inflammation},
author = {William J. Ennis and Audrey Sui and Amelia Bartholomew},
url = {https://doi.org/10.1016/j.fsc.2018.06.004},
doi = {https://doi.org/10.1016/j.fsc.2018.06.004},
year = {2013},
date = {2013-09-02},
urldate = {2013-09-02},
abstract = {Multipotent stem cells have paved the way for new applications and deeper understanding in regenerative medicine and the pathophysiology of aging. During skin aging, cumulative photodamage, exhaustion of endogenous stem cell populations, mechanical stress, and increased fibrosis lead to skin with decreased epidermal thickness and compromised dermal integrity. Specific stem cells in the hair follicle create new keratinocytes after activation by defensin peptides, released by neutrophils during wounding. Studies pertaining to defensin peptides' efficacy on skin aging have been published, highlighting their potential as a new therapy for skin rejuvenation.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
Wang, Xiuli Cong Lihua Wang Liming
Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy Journal Article
In: 2013.
@article{nokey,
title = {Human umbilical cord mesenchymal stem cell therapy for patients with active rheumatoid arthritis: safety and efficacy},
author = {Xiuli Cong Lihua Wang Liming Wang},
url = {https://doi.org/10.1089/scd.2013.0023},
year = {2013},
date = {2013-08-13},
urldate = {2013-08-13},
abstract = {This study was designed to assess the safety and efficacy of human umbilical cord mesenchymal stem cells (UC-MSCs) in the treatment of rheumatoid arthritis (RA). In this ongoing cohort, 172 patients with active RA who had inadequate responses to traditional medication were enrolled. Patients were divided into two groups for different treatment: disease-modifying anti-rheumatic drugs (DMARDs) plus medium without UC-MSCs, or DMARDs plus UC-MSCs group (4×107 cells per time) via intravenous injection. Adverse events and the clinical information were recorded. Tests for serological markers to assess safety and disease activity were conducted. Serum levels of inflammatory chemokines/cytokines were measured, and lymphocyte subsets in peripheral blood were analyzed. No serious adverse effects were observed during or after infusion. The serum levels of tumor necrosis factor-alpha and interleukin-6 decreased after the first UC-MSCs treatment (P<0.05). The percentage of CD4+CD25+Foxp3+ regulatory T cells of peripheral blood was increased (P<0.05). The treatment induced a significant remission of disease according to the American College of Rheumatology improvement criteria, the 28-joint disease activity score, and the Health Assessment Questionnaire. The therapeutic effects maintained for 3–6 months without continuous administration, correlating with the increased percentage of regulatory T cells of peripheral blood. Repeated infusion after this period can enhance the therapeutic efficacy. In comparison, there were no such benefits observed in control group of DMARDS plus medium without UC-MSCs. Thus, our data indicate that treatment with DMARDs plus UC-MSCs may provide safe, significant, and persistent clinical benefits for patients with active RA.},
keywords = {},
pubstate = {published},
tppubtype = {article}
}
REGENERATIVE MEDICINE
Complete the form to receive detailed information about regenerative medicine therapies for joint pain and osteoarthritis
- Secure HIPAA Compliant
- 48 Hours Response
- No Obligations
GETTING STARTED
Start by filling out our Pre Screening Questionnaire and a member of our team will reach out to discuss your interest and answer any initial questions.
PATIENT INTAKE QUESTIONNAIRE
Please fill out the patient intake questionnaire. This information will help our team better understand your medical history and needs.
SCHEDULE A VIDEO CONSULTATION
Book a video consultation with our medical team. During this consultation, you may be asked to provide any relevant medical records or images for review.
Final Review
Our team will conduct a final review of your case, and we will outline the recommended next steps based on your consultation and submitted information.

OmniStem Cell-based Therapies
Frequently Asked Questions
Please explore our Resource Center for helpful information. If you have any additional questions, feel free to contact our patient care team for assistance.
Umbilical cord mesenchymal stem cells (U-MSCs) are a type of adult stem cell found in Wharton’s jelly, a gelatinous substance within the umbilical cord. These cells have the ability to differentiate into various cell types, such as bone, cartilage, and fat cells, making them highly valuable for regenerative medicine and cell therapies.
Exosomes are microscopic, sac-like vesicles naturally released by cells, containing a rich payload of proteins, RNA, and other biomolecules essential for cellular communication and repair. Typically 30–200 nanometers in size, these extracellular vesicles play a pivotal role in maintaining and restoring cellular health.
At OmniStem, we harness the therapeutic potential of exosomes derived from umbilical cord mesenchymal stem cells (U-MSCs). These exosomes act as messengers, delivering regenerative signals to damaged tissues to support healing and reduce inflammation.
U-MSC stem cell treatments are considered specialized and are generally not covered by insurance plans.
U-MSC-derived allogenic stem cell therapies are currently not allowed in the U.S.
While mesenchymal stem cells (U-MSCs) have received FDA approval, the FDA does not permit the expansion of allogenic stem cells. As a result, patients undergoing these therapies in the U.S. typically receive a limited number of cells, which may reduce the potential benefits, including the use of U-MSC-derived exosomes.
At OmniStem, we use a safe and controlled laboratory process to expand these cells, enabling us to administer tens of millions of U-MSCs and U-MSC exosomes to our patients. This approach significantly enhances treatment outcomes.
This is a key reason many individuals travel to countries like Colombia, Panamá, Mexico, and the Dominican Republic to seek recognized providers of the most advanced regenerative medicine biologics available.
Our main facilities are located in the city of Pereira, in Colombia. We have specialized clinics and application centers for stem cell treatments that comply with all biosafety protocols and the highest safety standards. Pereira is the capital of the Colombian coffee region, one of the wonders of tourism in the country, and a World Heritage Site declared by UNESCO.









