NeuroBoost® U-MSC Derived Exosomes Protocol
Advancing Neurological Care with Exosome Therapy
Through the precise administration of umbilical cord-derived mesenchymal stem cell (U-MSC) exosomes via nebulization, NeuroBoost® introduces a cutting-edge approach to addressing neurological conditions. This non-invasive delivery method facilitates the targeted transport of therapeutic agents to the central nervous system, optimizing patient outcomes with minimal discomfort.
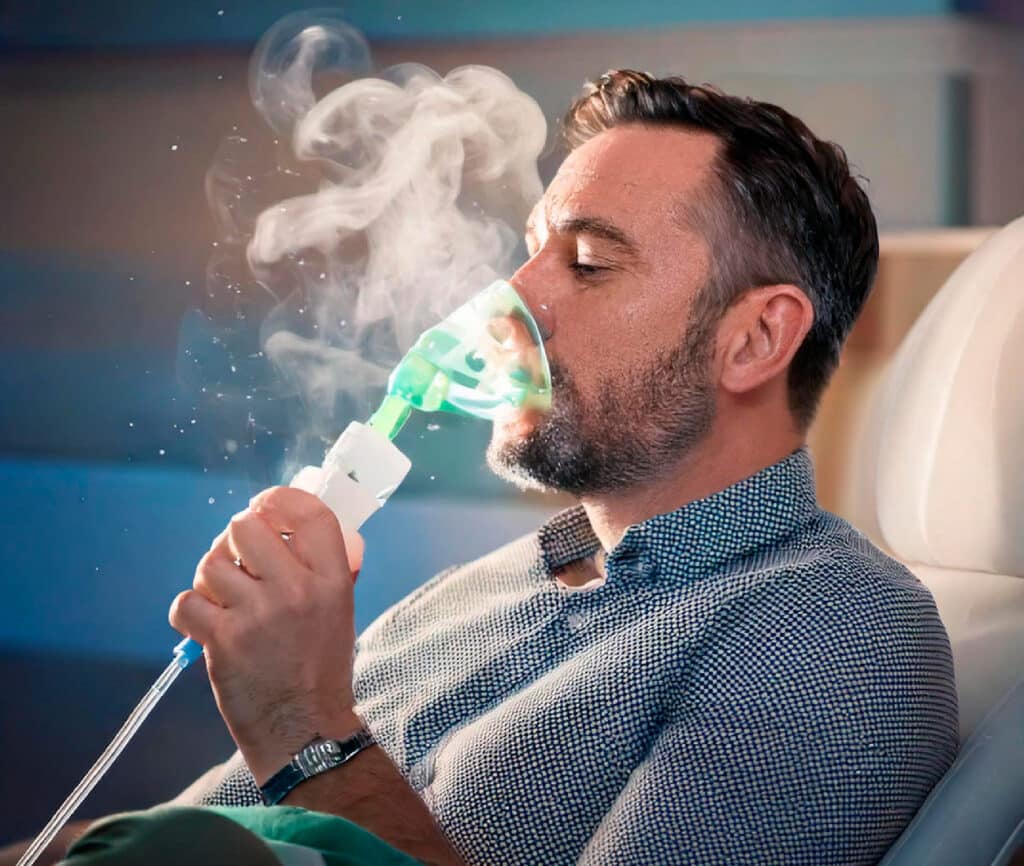
The Mechanism of Action: Science Behind NeuroBoost®
The central nervous system is safeguarded by the blood-brain barrier (BBB), a highly selective membrane that regulates molecular access to the brain. While the BBB protects neural tissue, it also poses a challenge for conventional therapies, many of which cannot effectively cross this barrier.
U-MSC-derived exosomes possess unique properties that enable them to cross the blood-brain barrier (BBB) via the cribriform plate. This property makes them particularly effective for delivering regenerative and neuroprotective signals directly to brain cells, aiding in the recovery from injuries and neurodegenerative conditions.
Clinical Advantages of NeuroBoost®
Anti-inflammatory Effects
Exosomes modulate neuroinflammation, a critical factor in many neurodegenerative and inflammatory brain diseases, by delivering cytokines and growth factors that help restore neural homeostasis.
Neuroprotection
Exosome therapy enhances cellular resilience and promotes the survival of neurons under stress, aiding recovery in conditions such as stroke, traumatic brain injury, and neurodegenerative disorders.
Functional Recovery
By stimulating neurogenesis and promoting synaptic repair, exosomes facilitate the restoration of cognitive and motor functions in affected patients.
Indications for NeuroBoost® Therapy
NeuroBoost® is designed for patients with a range of neurological challenges, including:
Neurodegenerative Disorders: Alzheimer’s disease, Parkinson’s disease, and amyotrophic lateral sclerosis (ALS).
Post-stroke Rehabilitation: Enhancing recovery of motor and cognitive abilities after ischemic or hemorrhagic stroke.
Neuroinflammatory Conditions: Conditions such as multiple sclerosis and autoimmune encephalitis.
Treatment Experience
Relaxed, Non-Invasive Procedure: You’ll sit comfortably in a modern clinic setting while the exosome therapy is administered through nebulization. This painless and non-invasive process typically takes less than an hour.
Advanced Cellular Delivery: Exosomes, rich in bioactive molecules, are delivered through a fine mist inhaled via your nose. These nanoparticles efficiently cross the blood-brain barrier, targeting areas of the brain that require support.
Take the Next Step in Neurological Health
Regain control over your neurological health with a cutting-edge treatment designed to repair and protect your brain. Contact us today to schedule a consultation and explore how NeuroBoost® can support your recovery and enhance your quality of life.
Complete the form to receive detailed information about NeuroBoost® U-MSC derived exosomes treatment.
- Secure HIPAA Compliant
- 48 Hours Response
- No Obligations
Prescreening Questionnaire
Start by filling out our Pre Screening Questionnaire to assess your eligibility for regenerative medicine treatments.
Video Consultation
Previous your consultation, complete a detailed medical questionnaire to finalize your treatment plan.
Patient Intake Questionnaire
Before your consultation, please complete a detailed medical questionnaire to finalize your treatment plan.
Final Review
Our team will review your case, create a personalized treatment plan, and provide a detailed quote for your treatment.

OmniStem Cell-based Therapies
Frequently Asked Questions
Please explore our Resource Center for helpful information. If you have any additional questions, feel free to contact our patient care team for assistance.
Umbilical cord mesenchymal stem cells (U-MSCs) are a type of adult stem cell found in Wharton’s jelly, a gelatinous substance within the umbilical cord. These cells have the ability to differentiate into various cell types, such as bone, cartilage, and fat cells, making them highly valuable for regenerative medicine and cell therapies.
Exosomes are microscopic, sac-like vesicles naturally released by cells, containing a rich payload of proteins, RNA, and other biomolecules essential for cellular communication and repair. Typically 30–200 nanometers in size, these extracellular vesicles play a pivotal role in maintaining and restoring cellular health.
At OmniStem, we harness the therapeutic potential of exosomes derived from umbilical cord mesenchymal stem cells (U-MSCs). These exosomes act as messengers, delivering regenerative signals to damaged tissues to support healing and reduce inflammation.
U-MSC stem cell treatments are considered specialized and are generally not covered by insurance plans.
U-MSC-derived allogenic stem cell therapies are currently not allowed in the U.S.
While mesenchymal stem cells (U-MSCs) have received FDA approval, the FDA does not permit the expansion of allogenic stem cells. As a result, patients undergoing these therapies in the U.S. typically receive a limited number of cells, which may reduce the potential benefits, including the use of U-MSC-derived exosomes.
At OmniStem, we use a safe and controlled laboratory process to expand these cells, enabling us to administer tens of millions of U-MSCs and U-MSC exosomes to our patients. This approach significantly enhances treatment outcomes.
This is a key reason many individuals travel to countries like Colombia, Panamá, Mexico, and the Dominican Republic to seek recognized providers of the most advanced regenerative medicine biologics available.
Our main facilities are located in the city of Pereira, in Colombia. We have specialized clinics and application centers for stem cell treatments that comply with all biosafety protocols and the highest safety standards. Pereira is the capital of the Colombian coffee region, one of the wonders of tourism in the country, and a World Heritage Site declared by UNESCO.


